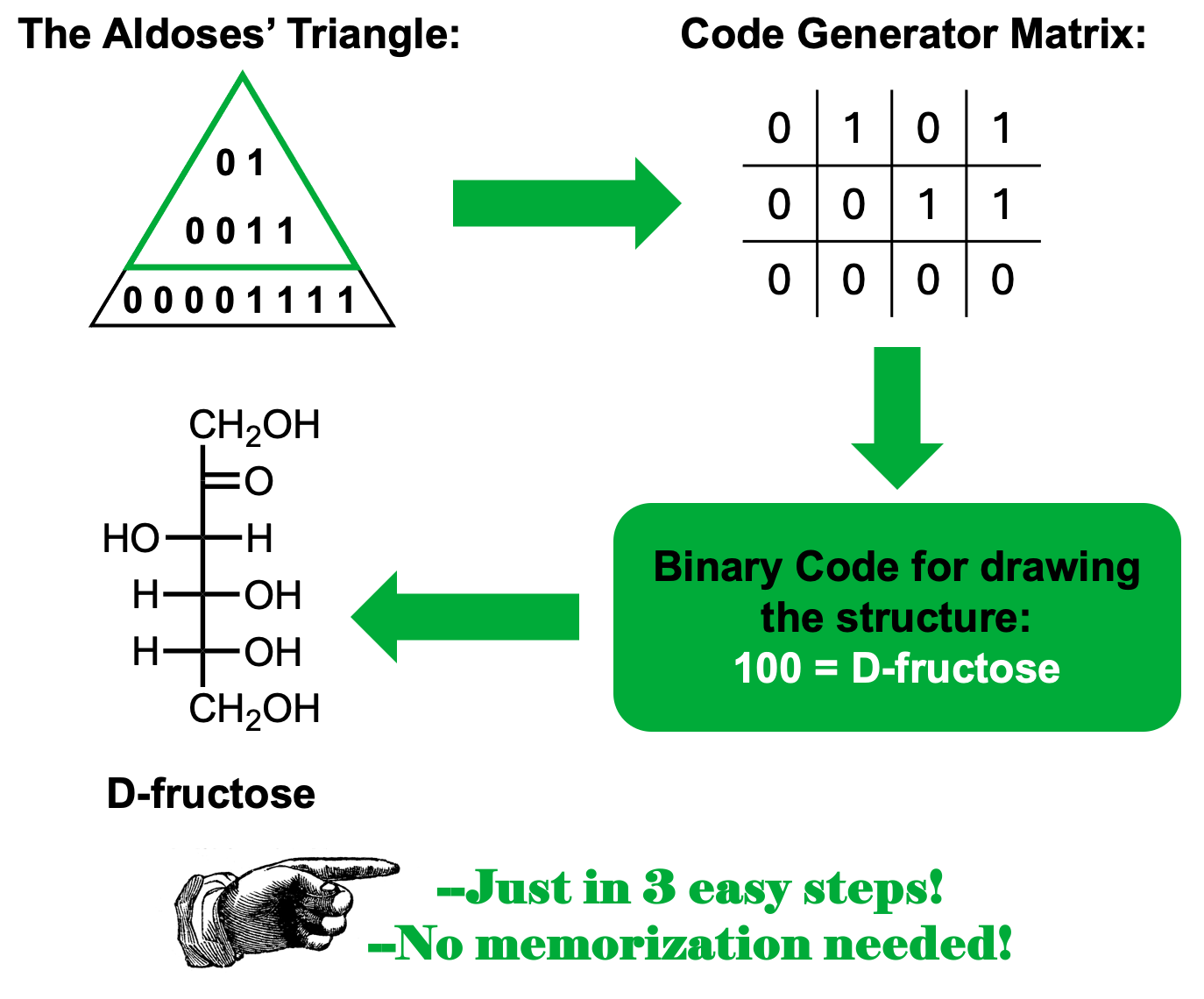
El triángulo de las aldosas
Contenido principal del artículo
Resumen
Una de las dificultades significativas que los estudiantes pueden encontrar al intentar aprobar un curso de Química Orgánica es la necesidad de memorizar mucha información. Esta práctica consume mucho tiempo y a veces el material memorizado se pierde durante los días siguientes a las evaluaciones. Dado a que el uso de mnemotecnias ha demostrado ser eficaz en la memorización a largo plazo, proponemos el uso del Triángulo de las Aldosas como una herramienta práctica cuando se tiene que lidiar con las estructuras de las aldopentosas, aldohexosas y cetohexosas.
Detalles del artículo
Citas en Dimensions Service
Citas
Arya, A., & Kumar, A. (2020). Teaching structural diversity of hexoses to graduate and postgraduate students: Methods to correlate stereochemistry. Biochemistry and Molecular Biology Education, 48(1), 8-20. https://doi.org/10.1002/bmb.21305
Butler, S. C. (2021). Push, pivot, and pull your way to converting Fischer projections into staggered bond-line structures. Journal of Chemical Education, 98(6), 2132-2137. https://doi.org/10.1021/acs.jchemed.1c00006
Carey, F. A., Giuliano, R. M., Allison, N. T., & Bane, S. L. (2018). Organic chemistry. McGraw-Hill Education.
Castillo, L., & Alvarez, L. X. (2015). Mnemotecnia para las aldosas y sus estructuras. Ciencia y Tecnología, 31(2), 21-27.
Deloach, W. S., & Brandon, A. (1955). A mnemonic aid for aldoses. Journal of Chemical Education, 32(8), 136. https://doi.org/10.1021/ed032p136
Fieser, L. F., & Fieser, M. (1959). Organic chemistry (3rd ed.). Reinhold.
Garrett, J. M. (1984). Brand the name with the linkage of the same. Journal of Chemical Education, 61(8), 665. https://doi.org/10.1021/ed061p665
Hallal, K., & Tlais, S. (2023). Arrow-rotation-method “ARM”, a simple and fast method for interconverting Fischer projections and zigzag structures. Journal of Chemical Education, 100(2), 991-997. https://doi.org/10.1021/acs.jchemed.2c01119
Klein, H. A. (1980). A simplified carbohydrate nomenclature. Journal of Chemical Information and Computer Sciences, 20, 15-18. https://doi.org/10.1021/ci60021a006
Leary, R. H. (1955). A mnemonic for the monosaccharides. Journal of Chemical Education, 32(8), 409. https://doi.org/10.1021/ed032p409
Levin, M. E., & Levin, J. R. (1990). Scientific mnemonomies: Methods for maximizing more than memory. American Educational Research Journal, 27(2), 301-321. https://doi.org/10.3102/00028312027002301
Mak, C. H. (2018). A simple paper model illustrates how to cyclize monosaccharides from Fischer projections to Haworth. Journal of Chemical Education, 95(8), 1336-1339. https://doi.org/10.1021/acs.jchemed.7b00832
Mastropieri, M. A., Emerci, K., & Scruggs, T. E. (1988). Mnemonic instruction of science concepts. Behavioral Disorders, 14(1), 48-56. https://doi.org/10.1177/019874298801400103
McGinn, C. J., & Wheatley, W. B. (1990). Binary representation in carbohydrate nomenclature. Journal of Chemical Education, 67(9), 747-748. https://doi.org/10.1021/ed067p747
McMurry, J. E. (2014). Organic chemistry with biological applications. Cengage Learning.
Mitschele, J. (1990). A mnemonic scheme for interconverting Fischer projections of open-chain monosaccharides and Haworth projections of corresponding α- and β-anomeric forms. Journal of Chemical Education, 67(7), 553. https://doi.org/10.1021/ed067p553
Moreno, L. F. (2012). Understanding Fischer projection and angular line representation conversion. Journal of Chemical Education, 89(11), 175-176. https://doi.org/10.1021/ed063p927
Neelakantan, S. (1969). Application of stereo numbers in sugar chemistry. Current Science, 38(15), 353-355.
Rosanoff, M. F. (1906). On Fischer’s classification of stereoisomers. Journal of the American Chemical Society, 28(1), 114–121. https://doi.org/10.1021/ja01967a014
Rosenblatt, D. H. (1965). A panoramic approach to the proof of configuration of aldohexoses. Journal of Chemical Education, 42(5), 271. https://doi.org/10.1021/ed042p271
Sattler, L. (1931). A number system for sugar configurations. Journal of Chemical Education, 8(7), 1369. https://doi.org/10.1021/ed008p1369
Starkey, R. (2000). SOS: A mnemonic for the stereochemistry of glucose. Journal of Chemical Education, 77(6), 734. https://doi.org/10.1021/ed077p734
Stewart, E. D. (1945). Association of names and formulas of the aldose sugars. Journal of Chemical Education, 22(4), 175-176. https://doi.org/10.1021/ed022p175
Wilson, J. L. (1988). Rules for determining D-, L- configurations in Haworth structures. Journal of Chemical Education, 65(9), 783. https://doi.org/10.1021/ed065p783
Yeoh, M. P. (2014). Musical mnemonics to facilitate learning of transcription of RNA. Learning Science and Mathematics, 9, 24-34.
Zheng, S. (2015). Mnemonics for the aldoses that aid in learning structures, names, and interconversion of Fischer projection formulas and pyranose chair forms. Journal of Chemical Education, 92(2), 395-398. https://doi.org/10.1021/ed500254x

Educación Química por Universidad Nacional Autónoma de México se distribuye bajo una Licencia Creative Commons Atribución-NoComercial-SinDerivar 4.0 Internacional.
Basada en una obra en http://www.revistas.unam.mx/index.php/req.