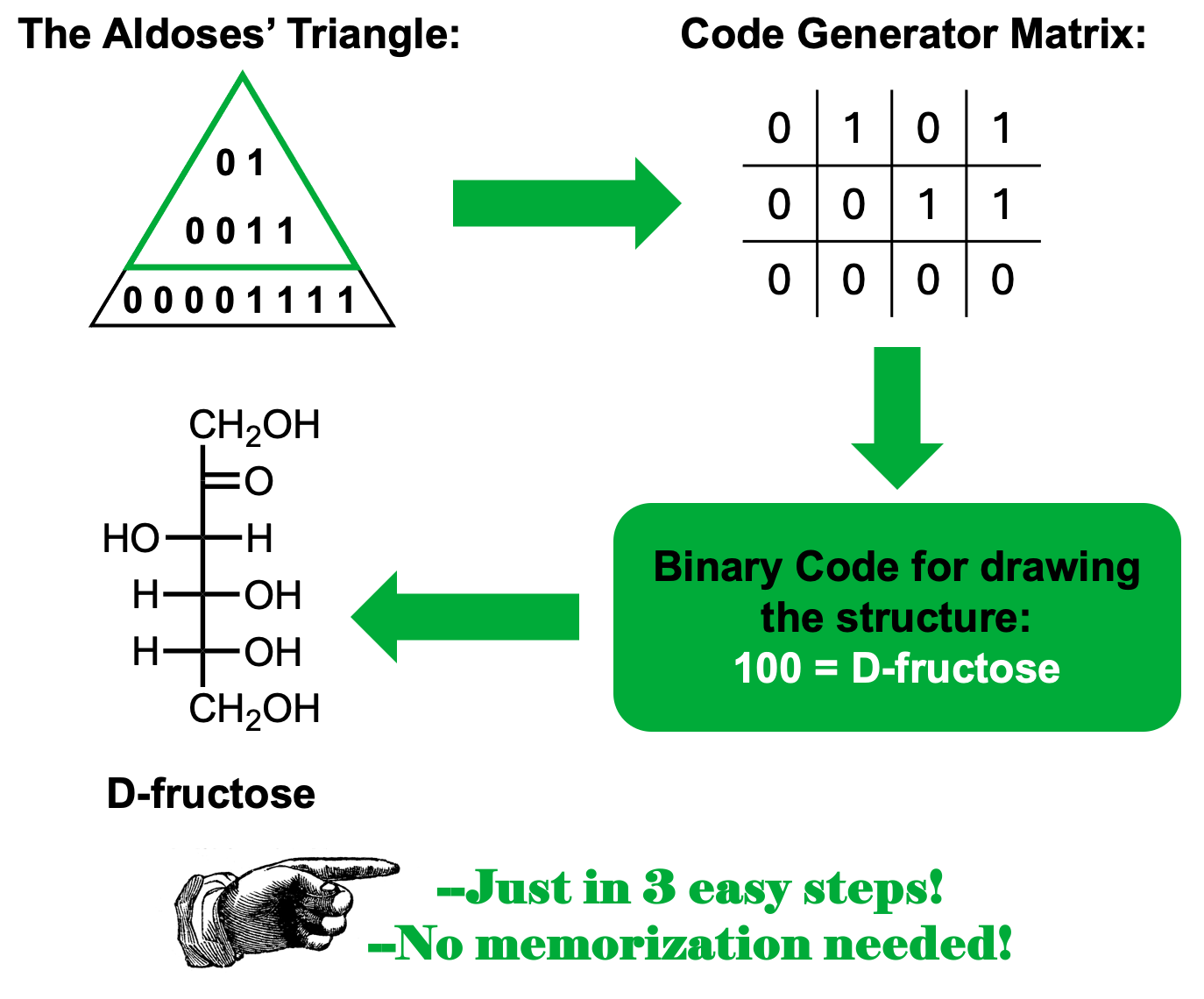
The aldoses’ triangle
Main Article Content
Abstract
One of the significant difficulties that students might find when trying to ace an Organic Chemistry course is the need to memorize a lot of information. This practice is very time-consuming and sometimes the memorized material is lost during the next few days after the evaluations. As the use of mnemonics has proven to be effective in long-term memorization, we propose the use of the Aldoses’ Triangle as a handy tool when dealing with the structures of the C5, C6 aldoses, and C6 ketoses.
Article Details
Citas en Dimensions Service
References
Arya, A., & Kumar, A. (2020). Teaching structural diversity of hexoses to graduate and postgraduate students: Methods to correlate stereochemistry. Biochemistry and Molecular Biology Education, 48(1), 8-20. https://doi.org/10.1002/bmb.21305
Butler, S. C. (2021). Push, pivot, and pull your way to converting Fischer projections into staggered bond-line structures. Journal of Chemical Education, 98(6), 2132-2137. https://doi.org/10.1021/acs.jchemed.1c00006
Carey, F. A., Giuliano, R. M., Allison, N. T., & Bane, S. L. (2018). Organic chemistry. McGraw-Hill Education.
Castillo, L., & Alvarez, L. X. (2015). Mnemotecnia para las aldosas y sus estructuras. Ciencia y Tecnología, 31(2), 21-27.
Deloach, W. S., & Brandon, A. (1955). A mnemonic aid for aldoses. Journal of Chemical Education, 32(8), 136. https://doi.org/10.1021/ed032p136
Fieser, L. F., & Fieser, M. (1959). Organic chemistry (3rd ed.). Reinhold.
Garrett, J. M. (1984). Brand the name with the linkage of the same. Journal of Chemical Education, 61(8), 665. https://doi.org/10.1021/ed061p665
Hallal, K., & Tlais, S. (2023). Arrow-rotation-method “ARM”, a simple and fast method for interconverting Fischer projections and zigzag structures. Journal of Chemical Education, 100(2), 991-997. https://doi.org/10.1021/acs.jchemed.2c01119
Klein, H. A. (1980). A simplified carbohydrate nomenclature. Journal of Chemical Information and Computer Sciences, 20, 15-18. https://doi.org/10.1021/ci60021a006
Leary, R. H. (1955). A mnemonic for the monosaccharides. Journal of Chemical Education, 32(8), 409. https://doi.org/10.1021/ed032p409
Levin, M. E., & Levin, J. R. (1990). Scientific mnemonomies: Methods for maximizing more than memory. American Educational Research Journal, 27(2), 301-321. https://doi.org/10.3102/00028312027002301
Mak, C. H. (2018). A simple paper model illustrates how to cyclize monosaccharides from Fischer projections to Haworth. Journal of Chemical Education, 95(8), 1336-1339. https://doi.org/10.1021/acs.jchemed.7b00832
Mastropieri, M. A., Emerci, K., & Scruggs, T. E. (1988). Mnemonic instruction of science concepts. Behavioral Disorders, 14(1), 48-56. https://doi.org/10.1177/019874298801400103
McGinn, C. J., & Wheatley, W. B. (1990). Binary representation in carbohydrate nomenclature. Journal of Chemical Education, 67(9), 747-748. https://doi.org/10.1021/ed067p747
McMurry, J. E. (2014). Organic chemistry with biological applications. Cengage Learning.
Mitschele, J. (1990). A mnemonic scheme for interconverting Fischer projections of open-chain monosaccharides and Haworth projections of corresponding α- and β-anomeric forms. Journal of Chemical Education, 67(7), 553. https://doi.org/10.1021/ed067p553
Moreno, L. F. (2012). Understanding Fischer projection and angular line representation conversion. Journal of Chemical Education, 89(11), 175-176. https://doi.org/10.1021/ed063p927
Neelakantan, S. (1969). Application of stereo numbers in sugar chemistry. Current Science, 38(15), 353-355.
Rosanoff, M. F. (1906). On Fischer’s classification of stereoisomers. Journal of the American Chemical Society, 28(1), 114–121. https://doi.org/10.1021/ja01967a014
Rosenblatt, D. H. (1965). A panoramic approach to the proof of configuration of aldohexoses. Journal of Chemical Education, 42(5), 271. https://doi.org/10.1021/ed042p271
Sattler, L. (1931). A number system for sugar configurations. Journal of Chemical Education, 8(7), 1369. https://doi.org/10.1021/ed008p1369
Starkey, R. (2000). SOS: A mnemonic for the stereochemistry of glucose. Journal of Chemical Education, 77(6), 734. https://doi.org/10.1021/ed077p734
Stewart, E. D. (1945). Association of names and formulas of the aldose sugars. Journal of Chemical Education, 22(4), 175-176. https://doi.org/10.1021/ed022p175
Wilson, J. L. (1988). Rules for determining D-, L- configurations in Haworth structures. Journal of Chemical Education, 65(9), 783. https://doi.org/10.1021/ed065p783
Yeoh, M. P. (2014). Musical mnemonics to facilitate learning of transcription of RNA. Learning Science and Mathematics, 9, 24-34.
Zheng, S. (2015). Mnemonics for the aldoses that aid in learning structures, names, and interconversion of Fischer projection formulas and pyranose chair forms. Journal of Chemical Education, 92(2), 395-398. https://doi.org/10.1021/ed500254x

Educación Química por Universidad Nacional Autónoma de México se distribuye bajo una Licencia Creative Commons Atribución-NoComercial-SinDerivar 4.0 Internacional.
Basada en una obra en http://www.revistas.unam.mx/index.php/req.